Your Location:Home >Produts >intermediates >606-23-5
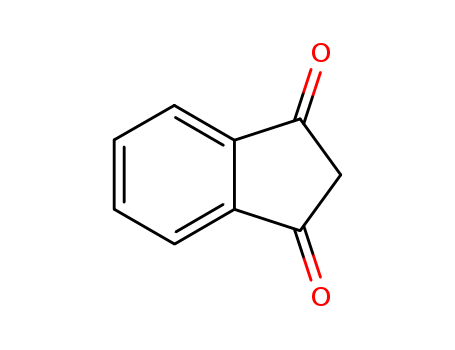

Product Details
|
Purification Methods |
Recrystallise it from EtOH or *C6H6. In dilute alkali it gives a deep yellow solution of the enol. [Bernasconi & Paschalis J Am Chem Soc 108 2969 1986]. [Beilstein 7 IV 2344.] |
|
Definition |
ChEBI: A member of the class of indanones that is indane in which the hydrogens at positions 2 and 4 have been replaced by oxo groups. |
InChI:InChI=1/C9H6O2/c10-8-5-9(11)7-4-2-1-3-6(7)8/h1-5,10H
A convenient and versatile procedure for...
The construction of C?O bonds through C?...
-
An efficient, practical and facile proce...
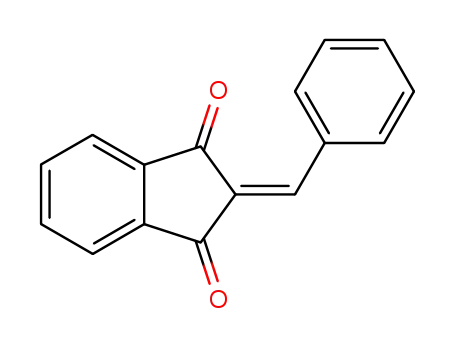
2-benzylidene-indan-1,3-dione

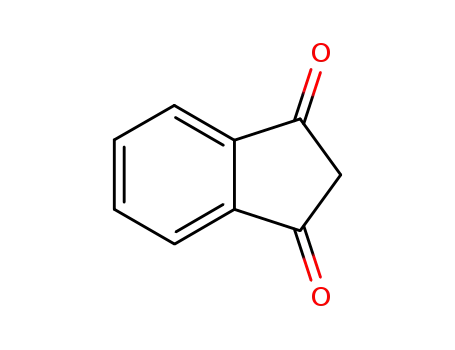
1H-indene-1,3(2H)-dione

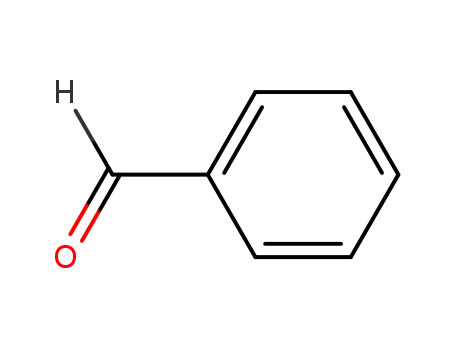
benzaldehyde
| Conditions | Yield |
|---|---|
|
water;
In
dimethyl sulfoxide;
at 20 ℃;
Rate constant;
Equilibrium constant;
effect of pH, buffer concentration;
|

2-benzylidene-indan-1,3-dione


1H-indene-1,3(2H)-dione


benzaldehyde
| Conditions | Yield |
|---|---|
|
|
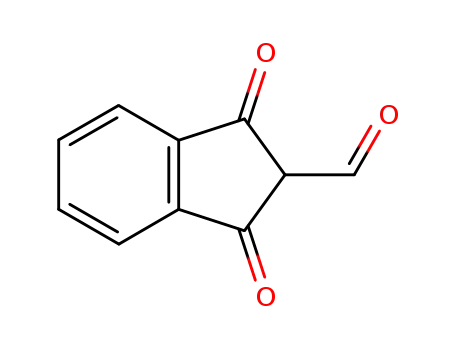
2-formylindane-1,3-dione
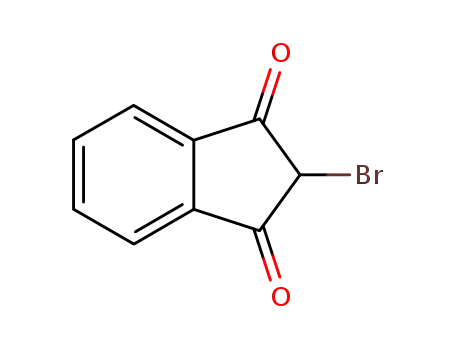
2-bromo-indan-1,3-dione
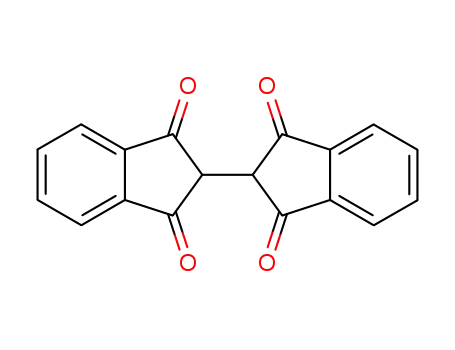
[2,2']biindenyl-1,3,1',3'-tetraone
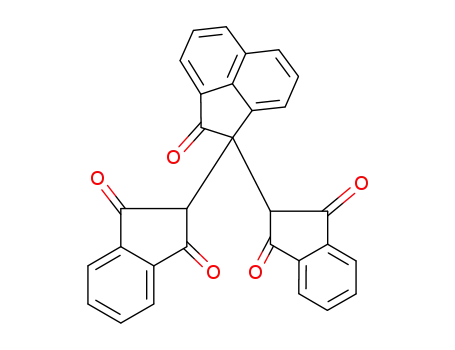
2,2-bis-(1,3-dioxo-indan-2-yl)-acenaphthen-1-one

2,2''-benzylidene-bis-[1,2']biindenylidene-3,1',3'-trione
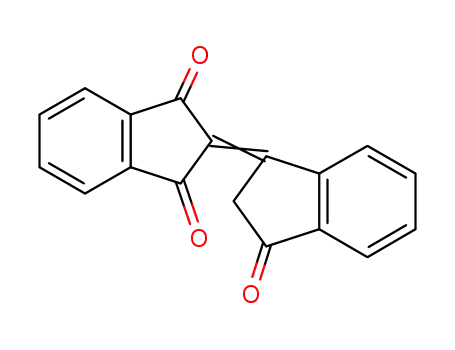
bindone
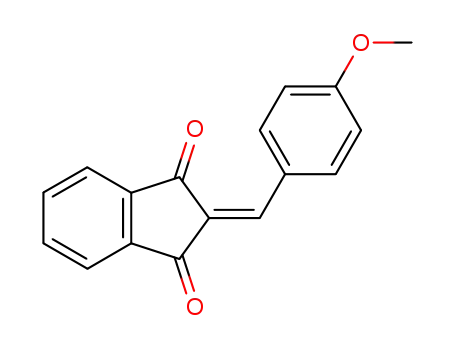
2-(4-methoxybenzylidene)indane-1,3-dione
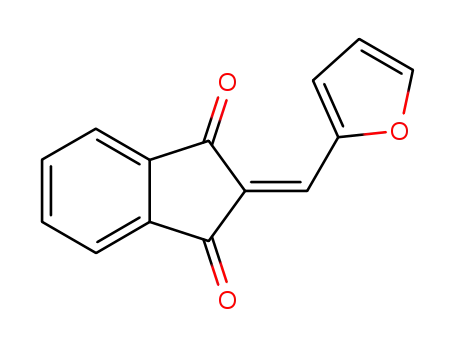
2-(2-furylmethylene)indane-1,3-dione