Your Location:Home >Produts >API >1421373-65-0

Product Details
Osimertinib, a third generation potent a...
The invention discloses a compound for i...
The present invention relates to an impr...
The invention relates to a preparation m...
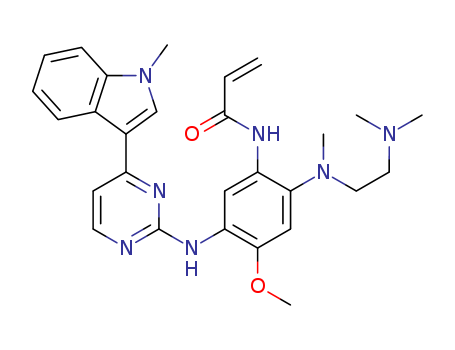
![N?2?[[2?(dimethylamino)ethyl]methylamino]?4?methoxy?5?[[4?(1?methyl?1H?indole-3-yl)-2-pyrimidinyl]amino]aniline](/upload/2025/6/bd50a060-375e-428a-a098-1a50782a611c.png)
N?2?[[2?(dimethylamino)ethyl]methylamino]?4?methoxy?5?[[4?(1?methyl?1H?indole-3-yl)-2-pyrimidinyl]amino]aniline

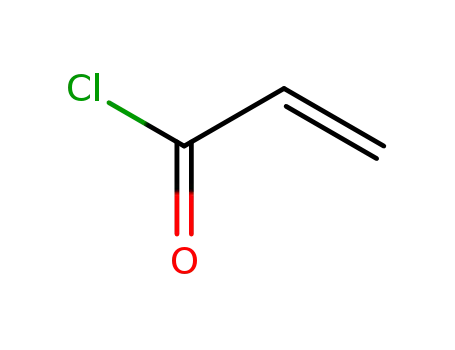
acryloyl chloride

amino}-4-methoxy-5-{[4-(1-methyl-1H-indol-3-yl)pyrimidin-2-yl]amino}phenyl)propen-2-amide]](/upload/2025/6/ce2ffc40-e370-46f5-bf31-26d7959cd035.png)
[N-(2-{[2-(dimethylamino)ethyl](methyl)amino}-4-methoxy-5-{[4-(1-methyl-1H-indol-3-yl)pyrimidin-2-yl]amino}phenyl)propen-2-amide]
| Conditions | Yield |
|---|---|
|
With
sodium carbonate;
In
N,N-dimethyl-formamide;
at -5 ℃;
for 2h;
Temperature;
Solvent;
|
95.5% |
|
With
N-ethyl-N,N-diisopropylamine;
In
tetrahydrofuran;
at 40 ℃;
for 12h;
Solvent;
Reagent/catalyst;
Temperature;
|
88% |
|
With
triethylamine;
In
tetrahydrofuran;
at 0 - 20 ℃;
for 3h;
Temperature;
Inert atmosphere;
|
87% |
|
With
triethylamine;
In
ethyl acetate;
at 5 - 10 ℃;
Inert atmosphere;
|
86.37% |
|
With
N-ethyl-N,N-diisopropylamine;
In
dichloromethane;
for 1.5h;
Cooling with ice;
|
83% |
|
With
triethylamine;
In
dichloromethane;
at 5 - 20 ℃;
|
79% |
|
With
N-ethyl-N,N-diisopropylamine;
In
chloroform;
at 5 - 15 ℃;
for 2h;
Solvent;
Temperature;
|
72% |
|
With
N-ethyl-N,N-diisopropylamine;
In
chloroform;
at 5 - 15 ℃;
for 2h;
Solvent;
|
72% |
|
With
N-ethyl-N,N-diisopropylamine;
In
dichloromethane;
for 1.5h;
Cooling with ice;
|
57.97% |
|
With
N-ethyl-N,N-diisopropylamine;
In
dichloromethane;
at -10 ℃;
for 1h;
|
47% |
|
With
N-ethyl-N,N-diisopropylamine;
In
dichloromethane;
for 1.5h;
Cooling with ice;
|
39% |
|
With
N-ethyl-N,N-diisopropylamine;
In
dichloromethane;
at 0 ℃;
for 1.5h;
Inert atmosphere;
|
39% |
|
N?2?[[2?(dimethylamino)ethyl]methylamino]?4?methoxy?5?[[4?(1?methyl?1H?indole-3-yl)-2-pyrimidinyl]amino]aniline;
With
N-ethyl-N,N-diisopropylamine;
In
dichloromethane;
at 0 ℃;
for 0.0833333h;
Inert atmosphere;
acryloyl chloride;
In
dichloromethane;
at 0 ℃;
Inert atmosphere;
|
34.2% |
![N?2?[[2?(dimethylamino)ethyl]methylamino]?4?methoxy?5?[[4?(1?methyl?1H?indole-3-yl)-2-pyrimidinyl]amino]aniline](/upload/2025/6/bd50a060-375e-428a-a098-1a50782a611c.png)
N?2?[[2?(dimethylamino)ethyl]methylamino]?4?methoxy?5?[[4?(1?methyl?1H?indole-3-yl)-2-pyrimidinyl]amino]aniline

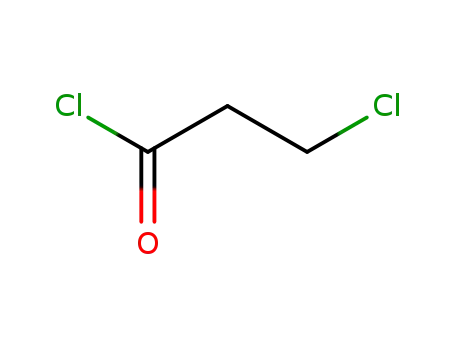
2-chloropropionyl chloride

amino}-4-methoxy-5-{[4-(1-methyl-1H-indol-3-yl)pyrimidin-2-yl]amino}phenyl)propen-2-amide]](/upload/2025/6/ce2ffc40-e370-46f5-bf31-26d7959cd035.png)
[N-(2-{[2-(dimethylamino)ethyl](methyl)amino}-4-methoxy-5-{[4-(1-methyl-1H-indol-3-yl)pyrimidin-2-yl]amino}phenyl)propen-2-amide]
| Conditions | Yield |
|---|---|
|
N?2?[[2?(dimethylamino)ethyl]methylamino]?4?methoxy?5?[[4?(1?methyl?1H?indole-3-yl)-2-pyrimidinyl]amino]aniline; 2-chloropropionyl chloride;
With
N-ethyl-N,N-diisopropylamine;
In
tetrahydrofuran; water;
at 0 - 20 ℃;
for 0.25h;
With
sodium hydroxide;
In
tetrahydrofuran; water;
at 65 ℃;
for 10h;
|
94% |
|
N?2?[[2?(dimethylamino)ethyl]methylamino]?4?methoxy?5?[[4?(1?methyl?1H?indole-3-yl)-2-pyrimidinyl]amino]aniline; 2-chloropropionyl chloride;
In
tetrahydrofuran; water;
at 20 ℃;
for 0.25h;
With
sodium hydroxide;
In
tetrahydrofuran; water;
at 65 ℃;
for 10h;
|
94% |
|
N?2?[[2?(dimethylamino)ethyl]methylamino]?4?methoxy?5?[[4?(1?methyl?1H?indole-3-yl)-2-pyrimidinyl]amino]aniline; 2-chloropropionyl chloride;
In
tetrahydrofuran;
at 0 - 20 ℃;
for 0.25h;
With
sodium hydroxide;
In
tetrahydrofuran;
at 65 ℃;
for 10h;
|
94% |
|
N?2?[[2?(dimethylamino)ethyl]methylamino]?4?methoxy?5?[[4?(1?methyl?1H?indole-3-yl)-2-pyrimidinyl]amino]aniline; 2-chloropropionyl chloride;
In
tetrahydrofuran; water;
at 0 - 28 ℃;
for 1h;
With
sodium hydroxide;
In
tetrahydrofuran; water;
for 8h;
Reflux;
|
79.6% |
|
N?2?[[2?(dimethylamino)ethyl]methylamino]?4?methoxy?5?[[4?(1?methyl?1H?indole-3-yl)-2-pyrimidinyl]amino]aniline; 2-chloropropionyl chloride;
In
water; acetone;
at -3 - 30 ℃;
for 1.5h;
With
triethylamine;
In
water; acetone;
at 25 - 60 ℃;
|
77.14% |
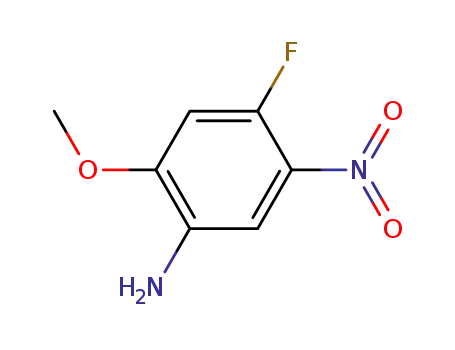
4-fluoro-2-methoxy-5-nitro-phenylamine
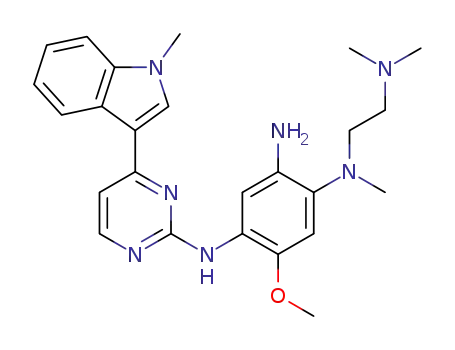
N?2?[[2?(dimethylamino)ethyl]methylamino]?4?methoxy?5?[[4?(1?methyl?1H?indole-3-yl)-2-pyrimidinyl]amino]aniline

acryloyl chloride

2-chloropropionyl chloride
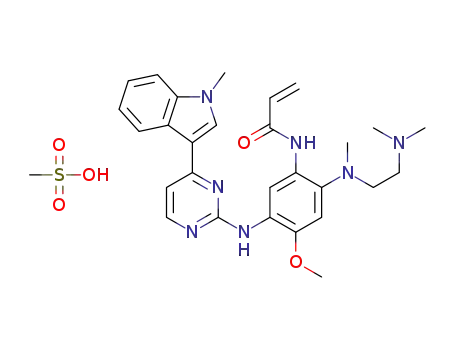
N-(2-(N-(2-(dimethylamino)ethyl)-N-methylamino)-4-methoxy-5-((4-(1-methyl-1H-indole-3-yl)pyrimidine-2-yl)amino)phenyl)acrylamide methanesulfonate
CAS:415918-91-1
Molecular Formula:C36H30NO2P
Molecular Weight:539.613