Your Location:Home >Produts >intermediates >719-22-2
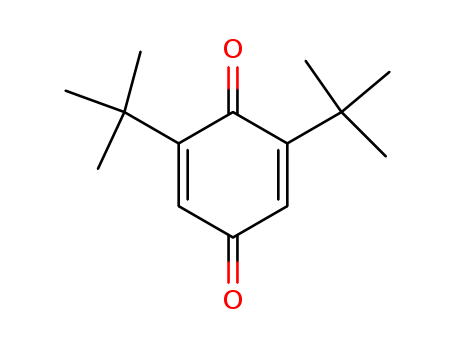

Product Details
|
General Description |
2,6-Di-tert-butylbenzoquinone is a chemical compound with the formula C14H22O2. It is a derivative of benzoquinone, which is a type of organic compound known for its aromatic properties and redox reactions. 2,6-Di-tert-butylbenzoquinone is a yellow crystalline solid that is sparingly soluble in water but highly soluble in organic solvents. It is mainly used as a polymerization inhibitor and antioxidant in the production of various polymers and plastics. Additionally, it also possesses anti-microbial and anti-fungal properties, making it useful in the formulation of certain personal care and cosmetic products. Overall, 2,6-Di-tert-butylbenzoquinone has a variety of industrial applications due to its stability and ability to hinder the degradation of polymers and other materials. |
InChI:InChI=1/C14H20O2/c1-13(2,3)10-7-9(15)8-11(12(10)16)14(4,5)6/h7-8H,1-6H3
Employing a strongly electron-donating t...
A convenient and high yielding preparati...
Two Co(II) complexes, Co(BBH)phenMeCN (1...
The stimulus to the modeling of enzyme f...
A systematic investigation into the favo...
A new oxime derivative N-heterocyclic hy...
Previous efforts to synthesize a cupric ...
Mn(II), Co(II), Cu(II), or Fe(III) ion c...
To evaluate the cytotoxic potential of m...
The oxygen affinities and properties of ...
Anion-exchange resins modified with meta...
The work in this paper presents the synt...
A cobalt(II) complex of 6,6'-bis(benzoyl...
Magnetic iron oxide nanoparticles coated...
The Ni(II) complex, {[Ni(HL)(SCN)2(H2O)]...
Instability of end-on superoxocopper(II)...
Selective oxidation of substituted pheno...
A mild photocatalytic phenol oxygenation...
We report herein a method for the oxidat...
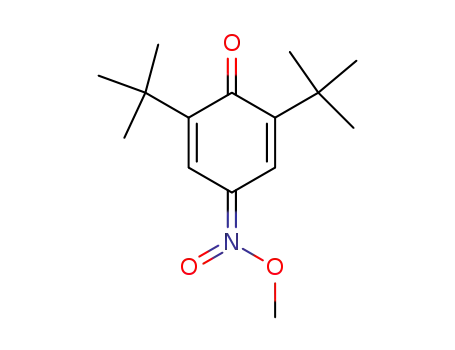
4-(methyl-aci-nitro)-2,6-di-t-butylcyclohexa-2,5-dienone

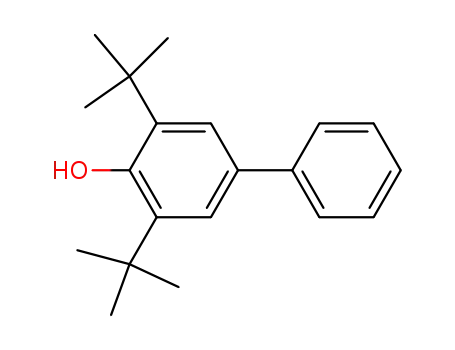
2,6-di(t-butyl)-4-phenylphenol

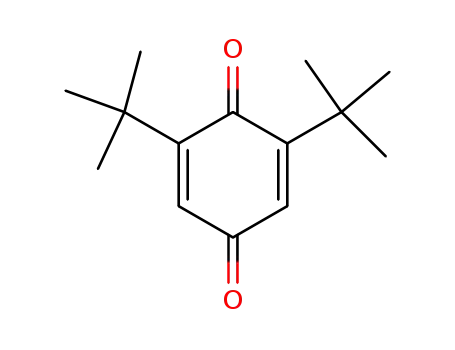
2,6-Di-tert-butyl-1,4-benzoquinone

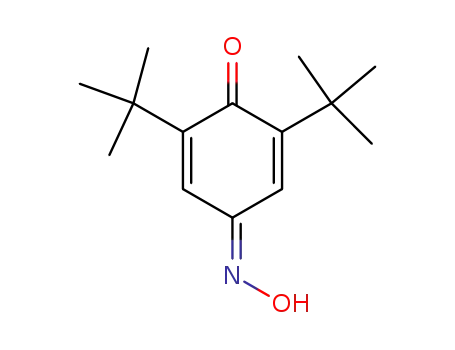
2,6-di-tert-butyl-1,4-benzoquinone monooxime

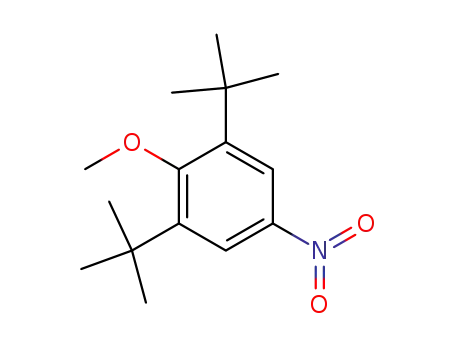
2-Methoxy-5-nitro-1,3-di-tert-butylbenzol

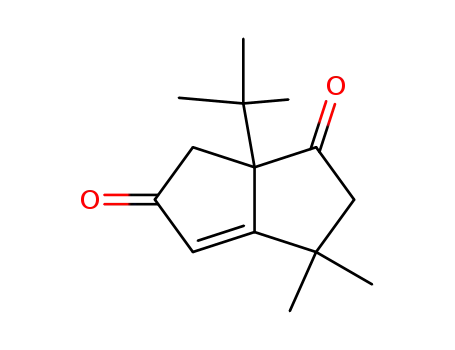
3,3,6,6a-tetrahydro-3,3-dimethyl-6a-t-butylpentalene-1,5-dione
| Conditions | Yield |
|---|---|
|
In
benzene;
at 20 ℃;
for 6h;
Product distribution;
Irradiation;
various nitronic esters under different conditions;
|
13.6% 3% 5% 10% 1% |

4-(methyl-aci-nitro)-2,6-di-t-butylcyclohexa-2,5-dienone


2,6-di(t-butyl)-4-phenylphenol


2,6-Di-tert-butyl-1,4-benzoquinone


2,6-di-tert-butyl-1,4-benzoquinone monooxime


3,3,6,6a-tetrahydro-3,3-dimethyl-6a-t-butylpentalene-1,5-dione
| Conditions | Yield |
|---|---|
|
In
benzene;
at 20 ℃;
for 6h;
Irradiation;
|
10% 65% 1% 3% |
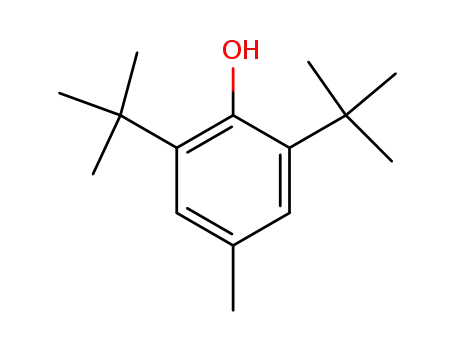
2,6-di-tert-butyl-4-methyl-phenol
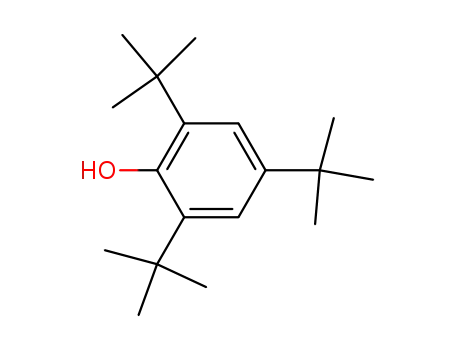
2,4,6-tri-tert-butylphenoxol
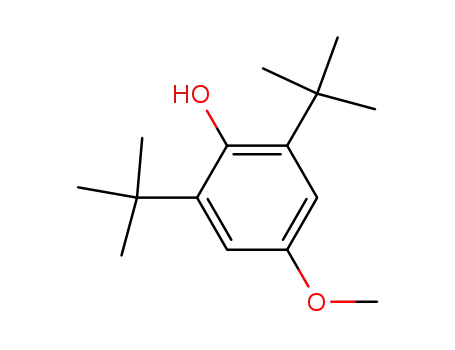
2,6-Di-t-butyl-4-methoxyphenol
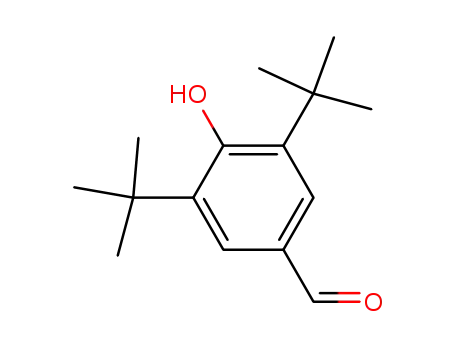
3,5-di-t-butyl-4-hydroxybenzaldehyde

2,6-Di-tert-butyl-4-phenylazo-phenol
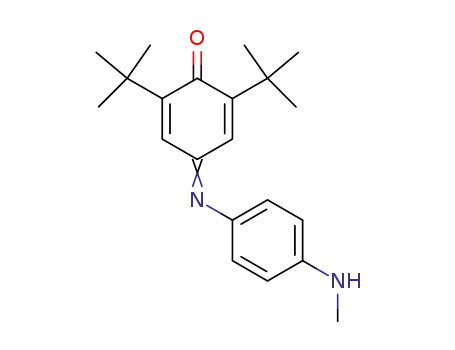
C21H28N2O
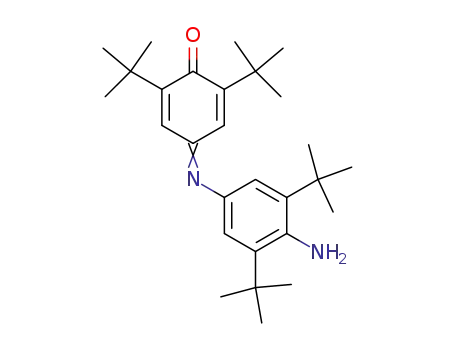
4-(4-Amino-3,5-di-tert-butyl-phenylimino)-2,6-di-tert-butyl-cyclohexa-2,5-dienone
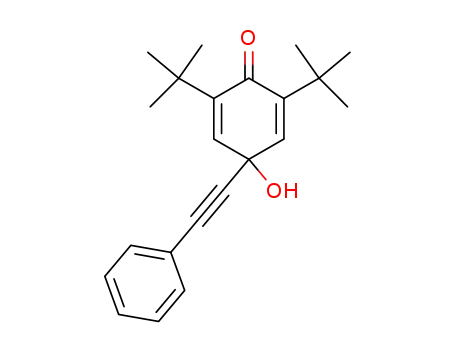
2,6-Di-tert-butyl-4-phenylaethinyl-p-hydrochinon
CAS:59718-84-2
Molecular Formula:C8H9NO2
Molecular Weight:151.165