Your Location:Home >Produts >intermediates >33529-02-1
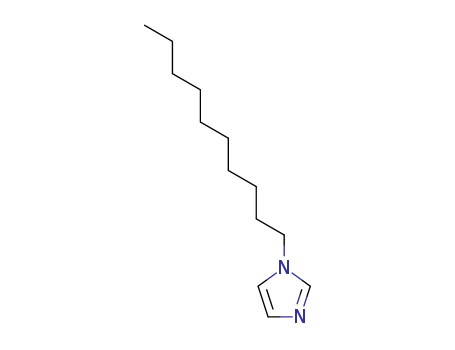

Product Details
|
General Description |
1-Decyl-1H-Imidazole is a chemical compound belonging to the class of organic compounds known as imidazoles. This type of compounds contains an imidazole ring, which is an unsaturated five-member ring structure with two nitrogen atoms and three carbon atoms. Imidazoles are regarded as fundamental units of various natural products and pharmaceuticals. The 1-Decyl-1H-Imidazole, specifically, is typically used in commercial applications and industrial products. However, detailed safety and toxicity information regarding its prolonged usage remain undisclosed due to limited studies. |
InChI:InChI=1/C13H24N2/c1-2-3-4-5-6-7-8-9-11-15-12-10-14-13-15/h10,12-13H,2-9,11H2,1H3
This paper presents the physicochemical ...
A series of imidazolium-based noncharged...
A novel alkylimidazolium iodide containi...
Lipase B from Candida antarctica (CAL-B)...
This article describes the ionic and sup...
Signal transduction is essential for the...
A series of compounds was designed and s...
Given the worldwide spread of bacterial ...
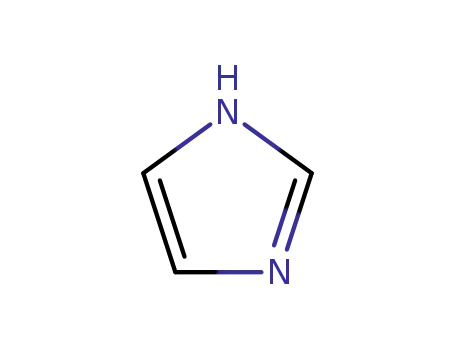
1H-imidazole


1-bromo dodecane

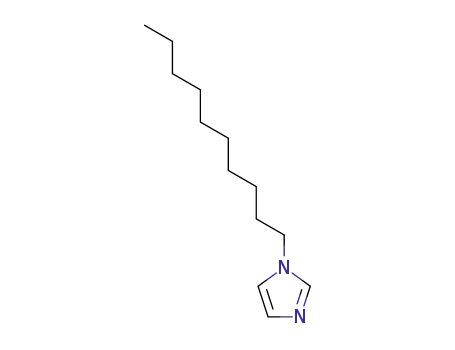
1-decylimidazole
| Conditions | Yield |
|---|---|
|
With
sodium hydroxide;
In
tetrahydrofuran;
for 72h;
Reflux;
|
98% |
|
With
tetrabutylammomium bromide; potassium carbonate; potassium hydroxide;
at 50 ℃;
Temperature;
Microwave irradiation;
|
94% |
|
With
sodium hydroxide;
In
dimethyl sulfoxide;
at 20 - 25 ℃;
Inert atmosphere;
|
88.3% |
|
1H-imidazole;
With
sodium hydride;
In
tetrahydrofuran; mineral oil;
at 0 ℃;
for 0.5h;
Inert atmosphere;
1-bromo dodecane;
In
tetrahydrofuran; mineral oil;
at 20 ℃;
Inert atmosphere;
|
86% |
|
With
propan-1-ol; sodium;
Heating;
|
85% |
|
1H-imidazole;
With
potassium hydroxide;
In
dimethyl sulfoxide;
at 20 ℃;
for 2h;
1-bromo dodecane;
In
dimethyl sulfoxide;
for 4h;
|
81.2% |
|
With
potassium hydroxide;
In
methanol; water;
at 90 ℃;
for 4h;
|
80% |
|
1H-imidazole;
With
sodium hydroxide;
In
dimethyl sulfoxide;
at 20 ℃;
for 1.5h;
1-bromo dodecane;
In
dimethyl sulfoxide;
at 20 ℃;
|
76% |
|
With
potassium carbonate;
In
acetone;
for 24h;
Reflux;
|
75% |
|
With
potassium carbonate;
In
N,N-dimethyl-formamide;
at 80 ℃;
for 12h;
Inert atmosphere;
|
71% |
|
With
sodium hydroxide; tetrabutylammomium bromide;
In
toluene;
at 60 - 80 ℃;
for 3h;
|
70% |
|
With
sodium hydroxide; tetraethylammonium iodide;
In
toluene;
for 10h;
Reflux;
|
64% |
|
With
tetraethylammonium iodide; sodium hydroxide;
In
toluene;
for 10h;
Reflux;
|
64% |
|
With
tetraethylammonium iodide; sodium hydroxide;
In
toluene;
for 10h;
Reflux;
|
64% |
|
With
sodium hydroxide; tetraethylammonium iodide;
In
toluene;
Heating;
|
63% |
|
With
1.) K;
Yield given. Multistep reaction;
1) THF, reflux, 2.) THF, reflux, 16 h;
|
|
|
With
sodium hydride;
In
tetrahydrofuran;
for 24h;
Heating;
|
|
|
With
potassium hydroxide; potassium carbonate;
tetrabutylammomium bromide;
for 0.0333333h;
microwave irradiation;
|
|
|
1H-imidazole;
With
sodium hydroxide;
In
tetrahydrofuran;
at 60 ℃;
for 1h;
1-bromo dodecane;
In
tetrahydrofuran;
at 60 ℃;
|
|
|
1H-imidazole;
With
sodium hydride;
In
tetrahydrofuran;
at 20 ℃;
for 1h;
1-bromo dodecane;
With
tetra-(n-butyl)ammonium iodide;
In
tetrahydrofuran;
at 20 ℃;
for 24h;
Inert atmosphere;
|
|
|
With
potassium carbonate;
In
acetone;
Reflux;
|
|
|
1H-imidazole; 1-bromo dodecane;
In
tetrahydrofuran;
at 20 ℃;
for 48h;
With
sodium hydroxide;
In
tetrahydrofuran;
for 12h;
|
|
|
1H-imidazole;
With
sodium methylate;
In
methanol;
1-bromo dodecane;
In
methanol;
at 65 ℃;
for 6h;
|
|
|
1H-imidazole;
With
potassium hydroxide;
In
dimethyl sulfoxide;
at 20 ℃;
for 0.5h;
1-bromo dodecane;
In
dimethyl sulfoxide;
at 18 ℃;
|
|
|
1H-imidazole;
With
sodium methylate;
for 8h;
1-bromo dodecane;
In
ethanol;
for 48h;
|
|
|
1H-imidazole;
With
sodium hydride;
In
1,4-dioxane;
at 90 ℃;
for 2h;
1-bromo dodecane;
In
1,4-dioxane;
at 90 ℃;
for 48h;
|
|
|
1H-imidazole;
With
potassium hydroxide;
In
dimethyl sulfoxide;
1-bromo dodecane;
In
dimethyl sulfoxide;
at 18 ℃;
for 2h;
|
|
|
With
potassium carbonate; potassium hydroxide;
In
acetonitrile;
at 80 ℃;
for 16h;
|
|
|
With
tetraethylammonium iodide; sodium hydroxide;
In
toluene;
for 10h;
Reflux;
|
|
|
1H-imidazole;
With
sodium;
In
ethanol;
for 2h;
Reflux;
Inert atmosphere;
1-bromo dodecane;
In
ethanol;
for 2h;
Reflux;
Inert atmosphere;
|
|
|
With
sodium hydroxide;
In
dimethyl sulfoxide;
at 20 ℃;
|

1H-imidazole

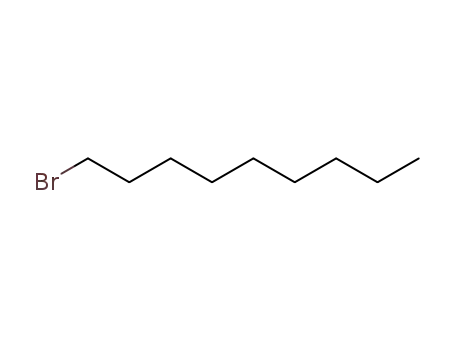
1-Bromononane


1-decylimidazole
| Conditions | Yield |
|---|---|
|
With
potassium carbonate;
In
acetone;
Reflux;
|
70% |

1H-imidazole
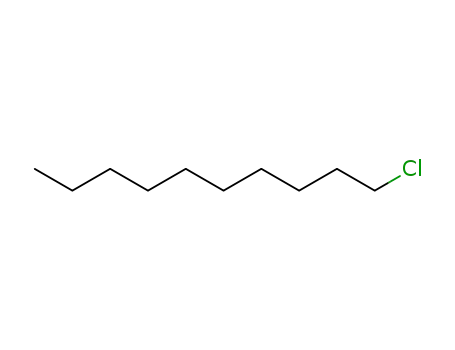
decyl chloride

1-bromo dodecane

Iododecane
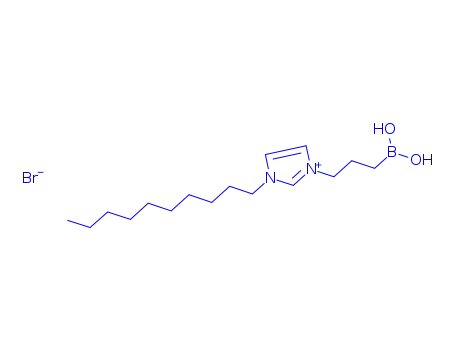
3-(3-decylimidazolium)propylboronic acid bromide

1-decyl-3-cyclohexylmethyl-1H-imidazolium bromide
CAS:36749-56-1
Molecular Weight:348.442