Your Location:Home >Produts >intermediates >21252-69-7
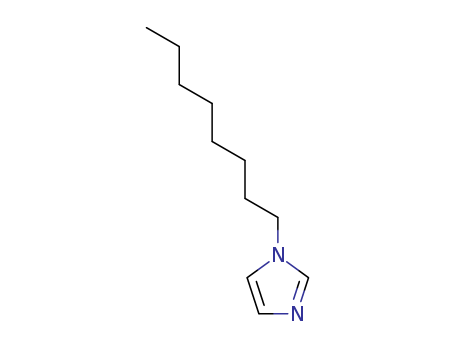

Product Details
|
General Description |
1-Octylimidazole is a widely used chemical compound with the molecular formula C12H21N2. It appears as a light yellow to brown clear liquid with a low melting and boiling point. 1-OCTYLIMIDAZOLE is primarily known for its role in chemical synthesis, particularly as a catalyst. Its solubility properties make it ideal for use in the manufacture of a variety of products ranging from pharmaceuticals to plastics. It is known for being stable under normal temperatures and pressures, however, it may pose certain health risks including skin and eye irritation or damage to organs through prolonged or repeated exposure. |
InChI:InChI=1/C11H20N2/c1-2-3-4-5-6-7-9-13-10-8-12-11-13/h8,10-11H,2-7,9H2,1H3
We synthesized a series of polyoxometala...
Keeping in mind the concept of green che...
Phase Transfer Catalysis in the absence ...
The design and synthesis of a series of ...
A series of compounds was designed and s...
1-(9-Anthracenylmethyl)-3-octylimidazoli...
Fluoroalkylated N-heterocyclic carbene c...
In order to overcome the hydrolysis of 2...
Two groups of disymmetric Gemini imidazo...
The aggregation of ionic liquid-based do...
The synthesis and characterization for a...
The novel synthesis of task-specific ion...
Structured semifluorinated polymer ionic...
Based on imidazolium and benzimidazolium...
A systematic study of the cellular uptak...
In the present study, four silver based ...
Signal transduction is essential for the...
The present disclosure relates to compos...
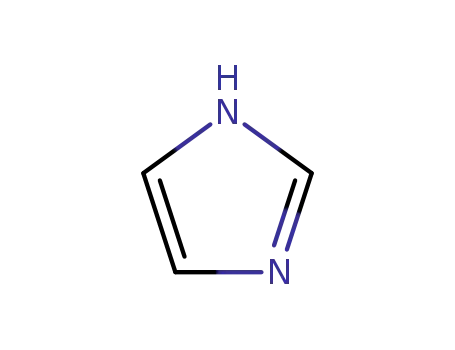
1H-imidazole

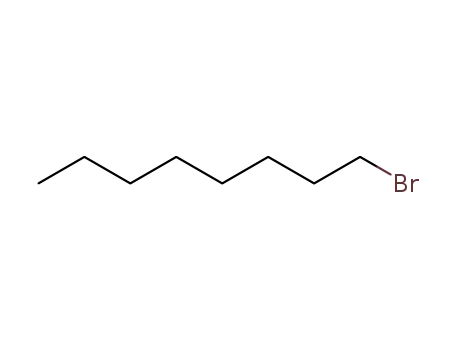
1-bromo-octane


N-octylimidazole
| Conditions | Yield |
|---|---|
|
With
potassium hydroxide;
at 80 ℃;
for 6h;
|
90% |
|
With
ethanol; sodium;
at 65 ℃;
for 20h;
|
90% |
|
1H-imidazole;
With
sodium hydroxide;
In
dimethyl sulfoxide;
at 90 ℃;
for 2h;
1-bromo-octane;
In
dimethyl sulfoxide;
at 20 - 65 ℃;
for 19h;
|
89% |
|
1H-imidazole;
With
sodium hydroxide;
In
dimethyl sulfoxide;
at 90 ℃;
for 2h;
1-bromo-octane;
In
dimethyl sulfoxide;
at 20 - 65 ℃;
for 19h;
|
89% |
|
With
sodium hydroxide;
In
dimethyl sulfoxide;
at 20 - 25 ℃;
Inert atmosphere;
|
87.7% |
|
With
sodium hydroxide;
In
tetrahydrofuran;
for 72h;
Reflux;
|
86% |
|
With
sodium hydroxide;
In
tetrahydrofuran; water;
Reflux;
|
85% |
|
With
propan-1-ol; sodium;
Heating;
|
83% |
|
1H-imidazole;
With
potassium hydroxide;
In
dimethyl sulfoxide;
at 20 ℃;
for 2h;
1-bromo-octane;
In
dimethyl sulfoxide;
for 4h;
|
82.3% |
|
1H-imidazole;
With
sodium hydride;
In
1,4-dioxane;
at 90 ℃;
for 1h;
1-bromo-octane;
In
1,4-dioxane;
at 90 ℃;
for 48h;
|
80% |
|
1H-imidazole;
With
potassium carbonate; potassium hydroxide;
In
dichloromethane;
for 0.25h;
1-bromo-octane;
With
tetra-(n-butyl)ammonium iodide;
In
dichloromethane;
|
75% |
|
In
toluene;
for 4h;
Reflux;
|
70% |
|
With
sodium hydroxide; tetraethylammonium iodide;
In
toluene;
Heating;
|
64% |
|
With
potassium carbonate;
In
acetone;
Reflux;
|
62% |
|
With
potassium carbonate;
In
acetone;
for 24h;
Reflux;
|
62% |
|
1H-imidazole;
With
sodium hydride;
In
tetrahydrofuran;
for 0.75h;
Inert atmosphere;
Cooling with ice;
1-bromo-octane;
In
tetrahydrofuran;
at 20 ℃;
for 24h;
Inert atmosphere;
|
60% |
|
1H-imidazole;
With
sodium hydrogencarbonate;
In
water; acetone;
at 25 ℃;
for 1h;
Inert atmosphere;
1-bromo-octane;
In
water; acetone;
at 50 ℃;
for 20h;
Inert atmosphere;
|
60% |
|
1H-imidazole;
With
potassium hydroxide;
In
methanol; water;
at 100 ℃;
1-bromo-octane;
In
methanol; water;
at 100 ℃;
for 3h;
|
50% |
|
In
ethyl acetate;
Heating;
|
|
|
With
sodium hydride;
In
tetrahydrofuran;
for 24h;
Heating;
|
|
|
With
potassium hydroxide; potassium carbonate;
tetrabutylammomium bromide;
for 0.0333333h;
microwave irradiation;
|
|
|
With
potassium carbonate;
In
acetone;
Reflux;
|
|
|
1H-imidazole; 1-bromo-octane;
In
tetrahydrofuran;
at 20 ℃;
for 48h;
With
sodium hydroxide;
In
tetrahydrofuran;
for 12h;
|
|
|
1H-imidazole;
With
sodium methylate;
In
methanol;
1-bromo-octane;
In
methanol;
at 65 ℃;
for 6h;
|
|
|
1-bromo-octane;
In
N,N-dimethyl-formamide;
1H-imidazole;
With
sodium hydride;
In
N,N-dimethyl-formamide;
at 60 ℃;
for 6h;
|
|
|
1H-imidazole;
With
potassium hydroxide;
In
dimethyl sulfoxide;
at 20 ℃;
for 0.5h;
1-bromo-octane;
In
dimethyl sulfoxide;
at 18 ℃;
|
|
|
1H-imidazole;
With
sodium methylate;
for 8h;
1-bromo-octane;
In
ethanol;
for 48h;
|
|
|
With
potassium hydroxide;
In
acetonitrile;
for 4h;
Reflux;
|
|
|
1H-imidazole;
With
potassium hydroxide;
In
dimethyl sulfoxide;
1-bromo-octane;
In
dimethyl sulfoxide;
at 18 ℃;
for 2h;
|
|
|
With
sodium hydroxide;
In
acetone;
at 20 ℃;
for 12h;
Reagent/catalyst;
|
|
|
With
potassium hydroxide;
In
N,N-dimethyl-formamide;
at 120 ℃;
for 16h;
Schlenk technique;
|
|
|
1H-imidazole;
With
sodium hydroxide;
In
tetrahydrofuran; methanol;
at 20 ℃;
for 1h;
1-bromo-octane;
In
tetrahydrofuran; methanol;
at 60 ℃;
for 16h;
|
|
|
1H-imidazole;
With
sodium hydroxide;
In
dimethyl sulfoxide;
at 90 ℃;
for 2h;
1-bromo-octane;
In
dimethyl sulfoxide;
at 20 - 65 ℃;
for 19h;
|
|
|
With
tetraethylammonium iodide; sodium hydroxide;
In
toluene;
for 10h;
Reflux;
|
|
|
1H-imidazole;
With
sodium;
In
ethanol;
for 2h;
Reflux;
Inert atmosphere;
1-bromo-octane;
In
ethanol;
for 2h;
Reflux;
Inert atmosphere;
|
|
|
With
potassium hydroxide;
In
dimethyl sulfoxide;
|

1H-imidazole

1-Bromoheptane


N-octylimidazole
| Conditions | Yield |
|---|---|
|
With
potassium carbonate;
In
acetone;
Reflux;
|
80% |
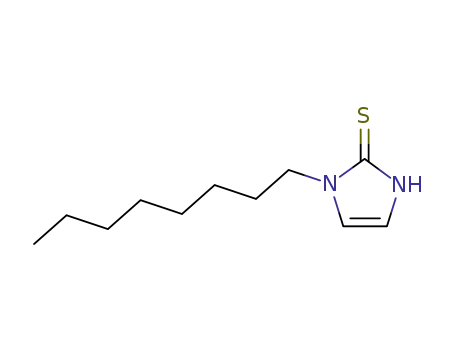
1-octyl-1,3-dihydro-imidazole-2-thione

1H-imidazole
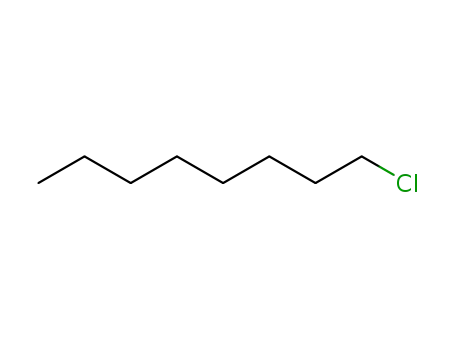
n-chlorooctane

1-bromo-octane
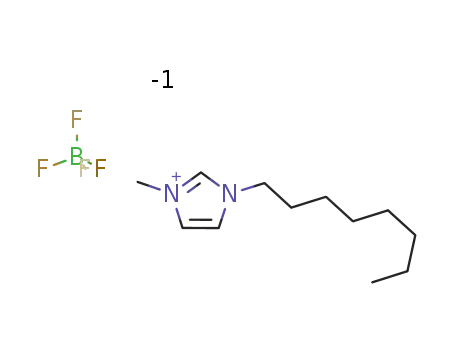
1-octyl-3-methylimidazolium tetrafluoroborate
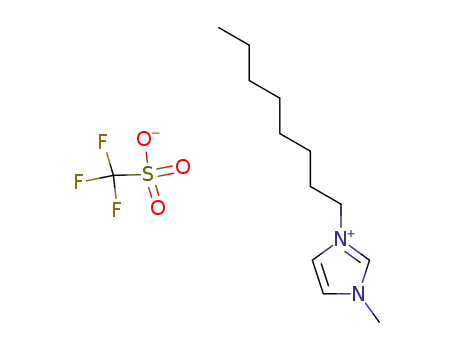
1-octyl-3-methyl-1H-imidazol-3-ium trifluoromethansulfonate
CAS:36749-56-1
Molecular Weight:348.442
CAS:2455-14-3
Molecular Formula:C28H40O2
Molecular Weight:408.624