Your Location:Home >Produts >intermediates >2455-14-3

Product Details
Use of dimethylformamide as solvent and ...
The electrochemical oxidation of selecte...
The unprecedented desymmetrization of pr...
Abstract: The hydrotalcite-like compound...
In order to explain the mechanism of the...
The oxygenation of 1,5-dihydroxynaphthal...
EPR-spectroscopic experiments of Co-Sale...
Crystals of the 3,3′-5,5′-tetra-tert-but...
A new class of mononuclear superoxocobal...
-
Recent breakthroughs have brought into q...
Good yields of 3,3′,5,5′-tetra-t-butyl-4...
In order to understand the effect that d...
Attempts have been made to synthesize gr...
-
The Cu(II) complexes, [Cu(2,5-pydc)(bmi)...
Although boron-containing radicals are p...
The stoichiometric oxidations of hydroqu...
The oxidative coupling reaction can effi...
The oxidation of 2,6-di-tert-butylphenol...
Fe(III)-EDTA in aq. MeOH offers a simple...
The facile preparative method of iron ox...
Cobalt phthalocyaninetetrasulfonate (CoP...
Long-chain N-alkyliminodiacetatomolybdat...
[1,6-Bis(2-hydroxyphenyl)-3,4-diaryl-2,5...
Pre-organization of Pd(II) species on po...
In recent years, it has become clear tha...
The H5PV2Mo10O40 heteropolyanion has bee...
In order to gain insight into the influe...
An efficient and simple method for the p...
-
The complex [FeIII2(μ-O2)(L3)4(S)2]4+(L3...
The heteroleptic (formazanato)nickel bro...
Phenoxyl radical was generally suggested...
An efficient oxidative NHC-catalyzed one...
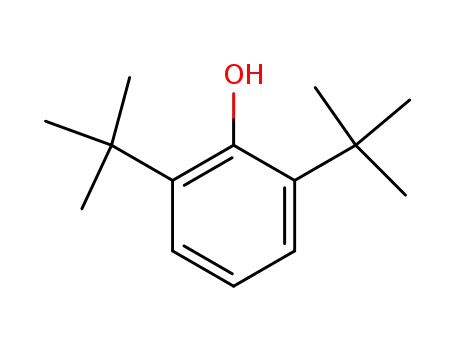
2,6-di-tert-butylphenol

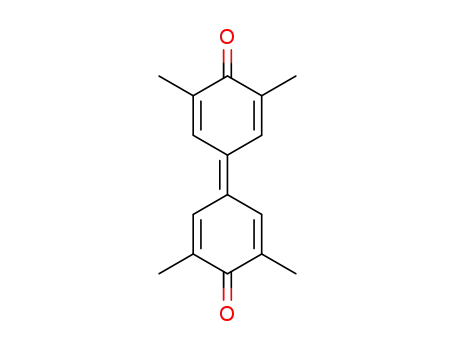
3,3',5,5'-tetramethyldiphenoquinone

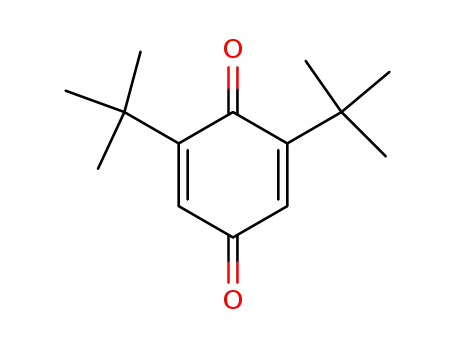
2,6-Di-tert-butyl-1,4-benzoquinone

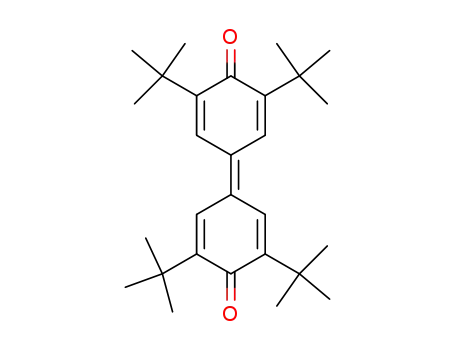
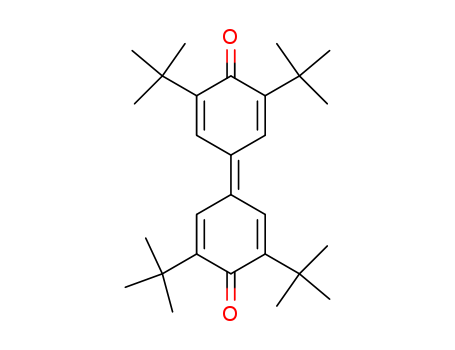
3,5,3',5'-tetra-tert-butyl-4,4'-diphenoquinone

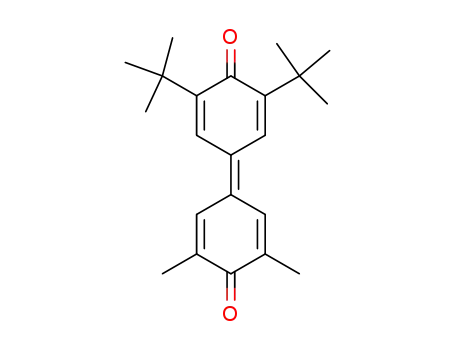
3,5-dimethyl-3',5'-di-t-butyl-4,4'-diphenoquinone
| Conditions | Yield |
|---|---|
|
With
2.6-dimethylphenol; manganese triacetate; acetic anhydride;
In
acetic acid;
at 100 ℃;
for 0.166667h;
Further byproducts given;
|
25% 14% 26% 10% |
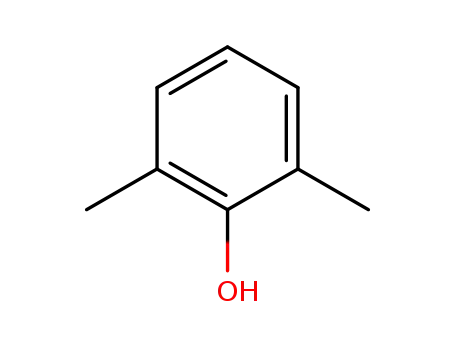
2.6-dimethylphenol


2,6-di-tert-butylphenol


3,3',5,5'-tetramethyldiphenoquinone


2,6-Di-tert-butyl-1,4-benzoquinone


3,5,3',5'-tetra-tert-butyl-4,4'-diphenoquinone


3,5-dimethyl-3',5'-di-t-butyl-4,4'-diphenoquinone
| Conditions | Yield |
|---|---|
|
With
manganese triacetate; acetic anhydride;
In
acetic acid;
at 100 ℃;
for 0.5h;
Further byproducts given;
|
25% 14% 10% 26% |

2,6-di-tert-butylphenol
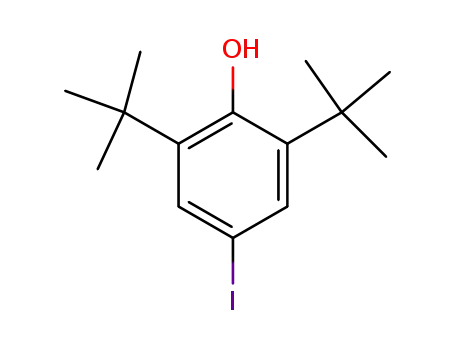
2,6-Di-tert-butyl-4-iodophenol
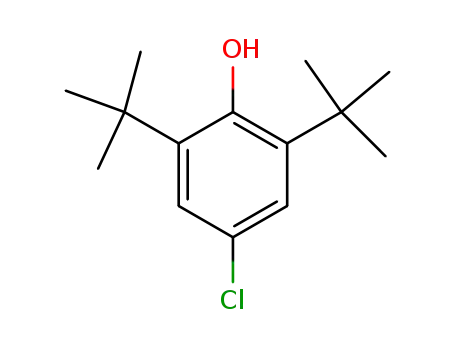
4-chloro-2,6-di-tert-butylphenol
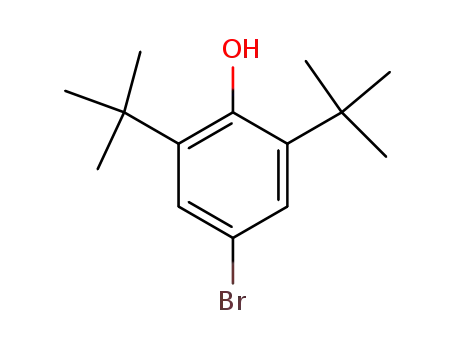
4-bromo-2,6-di-tert-butylphenol
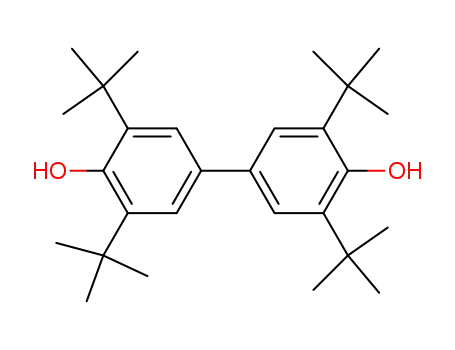
4,4'-dihydroxy-3,3',5,5'-tetra-tert-butylbiphenyl

2,6-di-tert-butylphenol

2,6-Di-tert-butyl-1,4-benzoquinone
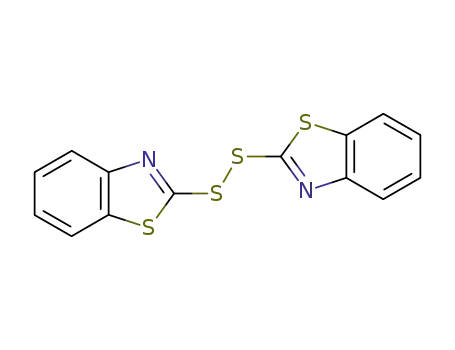
di(benzothiazol-2-yl)disulfide
CAS:40648-92-8
Molecular Formula:C16H19 N . Cl H
Molecular Weight:261.794