Your Location:Home >Produts >intermediates >40648-92-8

Product Details
InChI:InChI=1/C16H19N/c1-13(15-9-5-3-6-10-15)17-14(2)16-11-7-4-8-12-16/h3-14,17H,1-2H3/p+1/t13-,14-/m0/s1
The activation of amines by B(C6F5)3 is ...
The present invention relates to derivat...
An unusual class of chiral selectors, cy...
Stereospecific lithiation of N-α-methylb...
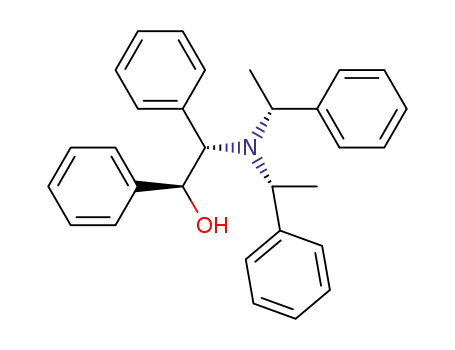
(2R, 4S, 5S, 3'R)-(+)-N,N-bis(1-phenylethyl)-1,2-diphenyl-2-aminoethanol

![(R,R)-(+)-bis[alpha-methylbenzyl]amine hydrochloride](/upload/2025/6/b246a9ee-572c-4bb4-9525-1ddcb0b06e9b.png)
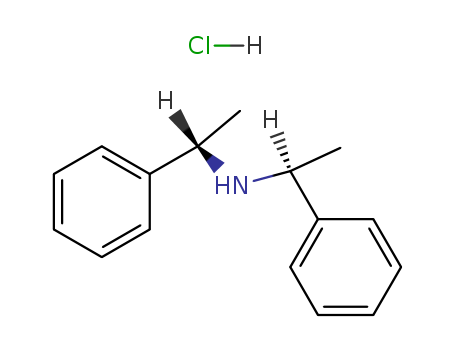
(R,R)-(+)-bis[alpha-methylbenzyl]amine hydrochloride
| Conditions | Yield |
|---|---|
|
With
lead(IV) acetate;
In
benzene;
at 50 - 60 ℃;
for 5h;
|
48.5% |
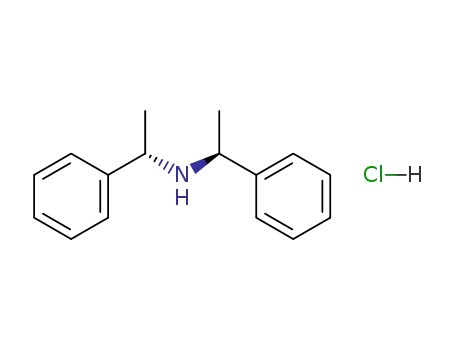
bis(1-phenylethyl)amine hydrochloride

![(R,R)-(+)-bis[alpha-methylbenzyl]amine hydrochloride](/upload/2025/6/b246a9ee-572c-4bb4-9525-1ddcb0b06e9b.png)
(R,R)-(+)-bis[alpha-methylbenzyl]amine hydrochloride

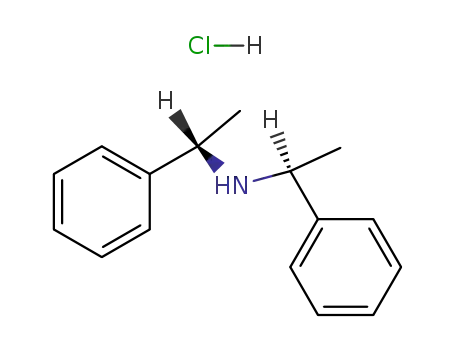
hydrochloride salt of (1S,1'S)-bis(1-phenylethyl)amine
| Conditions | Yield |
|---|---|
|
With
N-[(R)-1-(1-naphthyl)ethyl]carbamoyl-derivatized cyclofructan-6 column;
In
ethanol; n-heptane; trifluoroacetic acid;
at 20 ℃;
Resolution of racemate;
|
methyl magnesium iodide
(2R, 4S, 5R, 3'R)-(-)-N-(1-phenylethyl)-2,4,5-triphenyloxazolidine

(2R, 4S, 5S, 3'R)-(+)-N,N-bis(1-phenylethyl)-1,2-diphenyl-2-aminoethanol
(2R, 4R, 5S, 3'R)-N,N-bis(1-phenylethyl)-1,2-diphenyl-2-aminoethanol
4-methoxy-N,N-bis-(1-phenyl-ethyl)-benzamide
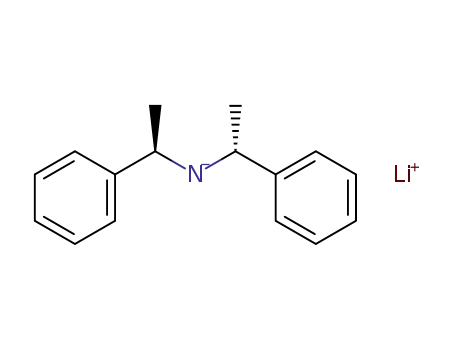
lithium [R(R*,R*)]-(+)-bis(α-methylbenzyl)amide
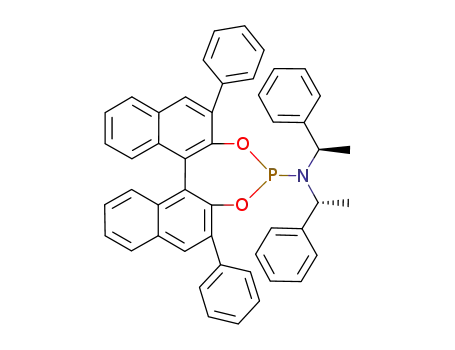
C48H38NO2P
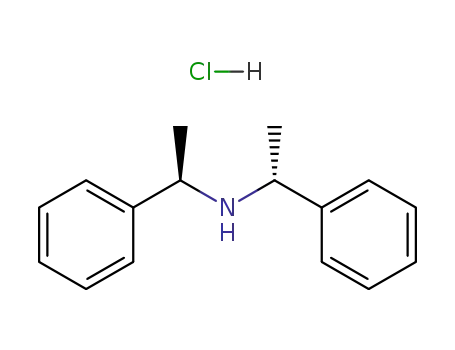
(R,R)-(+)-bis[alpha-methylbenzyl]amine hydrochloride
CAS:2455-14-3
Molecular Formula:C28H40O2
Molecular Weight:408.624
CAS:82398-30-9
Molecular Formula:C16H19 N . Cl H
Molecular Weight:261.794