Your Location:Home >Produts >API >857890-39-2

Product Details
Bulk supply high purity Lenvatinib Mesylate 857890-39-2, Paid sample available We provide high quality lenvatinib Mesylate (CAS 857890-39-2), at a very affordable prices to our customers.
Lenvatinib mesylate (lenvatinib) is an orally available, receptor‐type tyrosine kinase inhibitor, which was developed at Eisai in 2015. It was approved by the FDA in 2015 for the treatment of differentiated thyroid cancer that is either locally recurrent, metastatic, or progressive and did not respond to radioactive iodine treatment. In May 2016, the FDA approved the drug as a combination therapy with everolimus for the treatment of advanced renal cell carcinoma. Because VEGF (and fibroblast growth factor receptors, known as FGFRs) are thought to play a role in cardiovascular signaling pathways, VEGF2R and FGFR inhibition are thought to be the mechanisms behind the primary side effect of lenvatinib mesylate, which is hypertension.
Lenvatinib Mesylate is used in preparation of anti-human CTLA4xPD-1 bispecific antibodies for diagnosis, prevention and treatment of tumor or anemia.
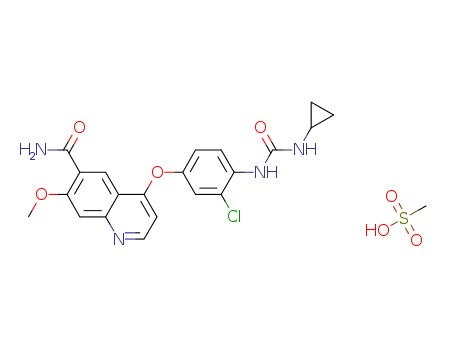
The CAS number of lenvatinib Mesylate is 857890-39-2.
More information of lenvatinib Mesylate 857890-39-2 are:
|
CAS Number |
857890-39-2 |
|
PSA |
178.32000 |
|
LogP |
5.82090 |
The chemical formula of lenvatinib Mesylate is C21H19ClN4O4.CH4O3S which containing 22 Carbon atoms,23 Hydrogen atoms,1 Chlorine atoms,4 Nitrogen atoms,4 Oxygen atoms and 1 Sulfur atoms,and the molecular weight, and the molecular weight of lenvatinib Mesylate is 522.966
Lenvatinib mesylate (lenvatinib) is an orally available, receptor‐type tyrosine kinase inhibitor, which was developed at Eisai in 2015. It was approved by the FDA in 2015 for the treatment of differentiated thyroid cancer that is either locally recurrent, metastatic, or progressive and did not respond to radioactive iodine treatment. In May 2016, the FDA approved the drug as a combination therapy with everolimus for the treatment of advanced renal cell carcinoma. Because VEGF (and fibroblast growth factor receptors, known as FGFRs) are thought to play a role in cardiovascular signaling pathways, VEGF2R and FGFR inhibition are thought to be the mechanisms behind the primary side effect of lenvatinib mesylate, which is hypertension.
Relevant articles related to lenvatinib Mesylate:
|
Article |
Source |
|
Novel method for the synthesis of lenvatinib using 4-nitrophenyl cyclopropylcarbamate and their pharmaceutical salts |
Sadineni, Ravi Kumar,Rapolu, Rajesh Kumar,Raju, V. V. N. K. V. Prasada,Srinivasu,Malladi, Sireesha,Mulakayala, Naveen , p. 1475 - 1483 (2020/11/05) |
Luhan Pharmachem Co., Ltd. is a quality supplier of lenvatinib Mesylate. Our main goal is customer satisfaction. Contact us to negotiate the best price for your business on lenvatinib Mesylate 857890-39-2.