Your Location:Home >Produts >intermediates >3034-50-2
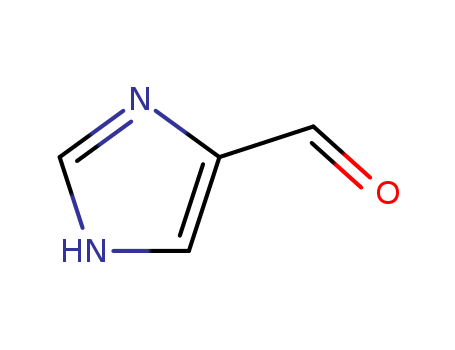

Product Details
|
General Description |
1H-Imidazole-4-carbaldehyde is a versatile intermediate in organic synthesis, accessible through various methods such as regioselective N-alkylation of 4-formylimidazole, photooxidative cleavage of urocanic acid, and efficient synthetic routes involving lithiation and formylation. Its reactivity and applications are highlighted by its role in selective alkylation reactions, photochemical transformations, and as a precursor for N-protected derivatives. |
InChI:InChI=1/C5H6N2O/c1-4(8)5-2-6-3-7-5/h2-3H,1H3,(H,6,7)
We describe here a high yield and highly...
Urocanic acid undergoes photooxidative c...
Successive addition of BuLi, triethylsil...
-
The invention discloses a method for pre...
The invention discloses a synthesis meth...
The present disclosure provides conjugat...
A selective and practical bromine–metal ...
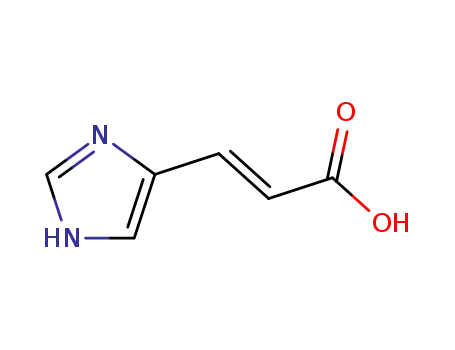
urocanic Acid

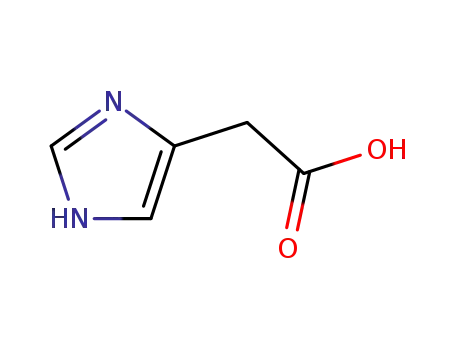
2-(1H-imidazol-4-yl)acetic acid

4(5)formylimidazole

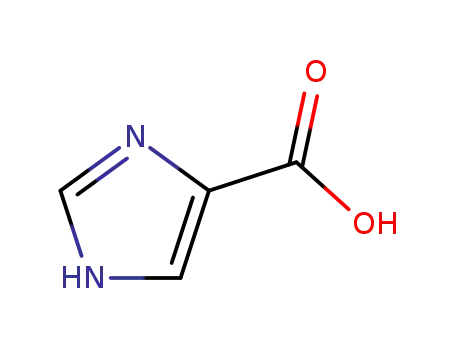
imidazole-4-carboxylic acid
| Conditions | Yield |
|---|---|
|
With
dihydrogen peroxide;
pH=7.2;
Product distribution / selectivity;
aqueous phosphate buffer;
|
|
|
With
dihydrogen peroxide;
In
water;
pH=7.2;
Product distribution / selectivity;
|

urocanic Acid


2-(1H-imidazol-4-yl)acetic acid

4(5)formylimidazole


imidazole-4-carboxylic acid

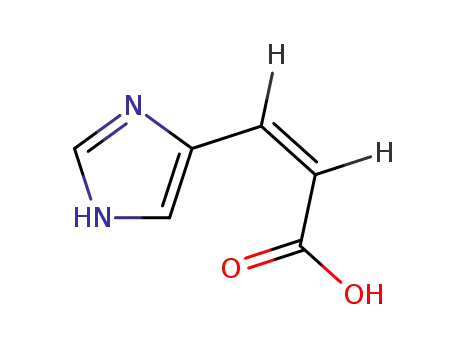
(Z)-urocanic acid
| Conditions | Yield |
|---|---|
|
With
dihydrogen peroxide;
pH=7.2;
Product distribution / selectivity;
aqueous phosphate buffer;
UV-irradiation;
|
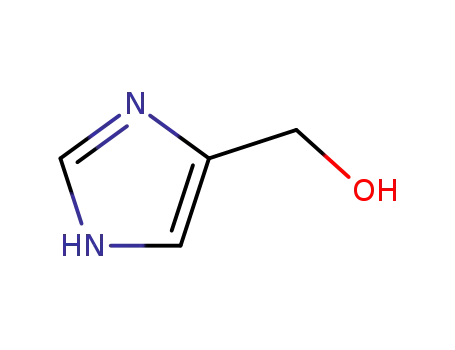
(1H-imidazol-4-yl)methanol
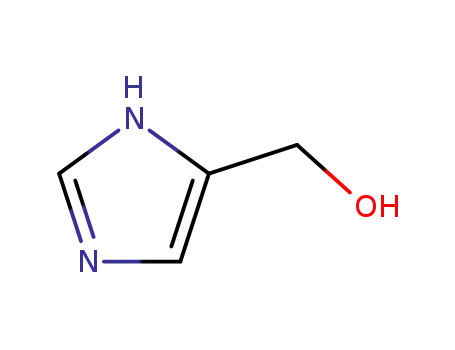
1H-imidazole-5-methanol
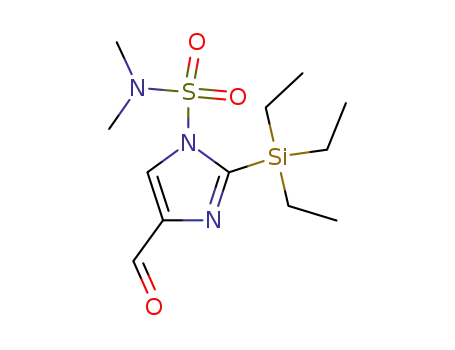
1-(N,N-dimethylsulfamoyl)-2-(triethylsilyl)imidazole-4-carbaldehyde
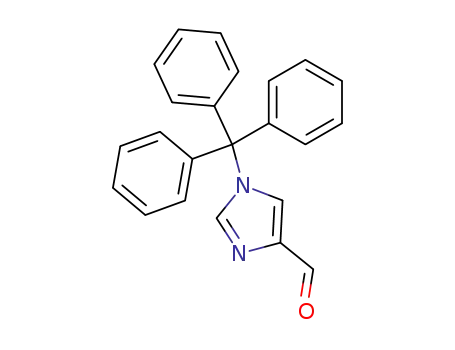
(1-tritylimidazol-4-yl)carboxaldehyde

urocanic Acid
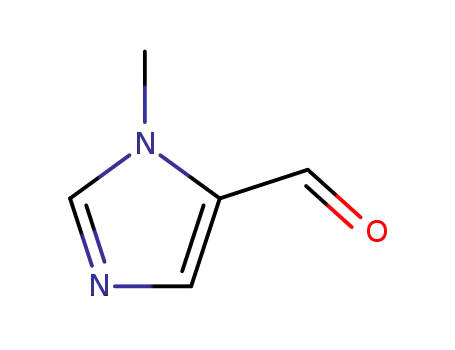
1-methylimidazole-5-carbaldehyde
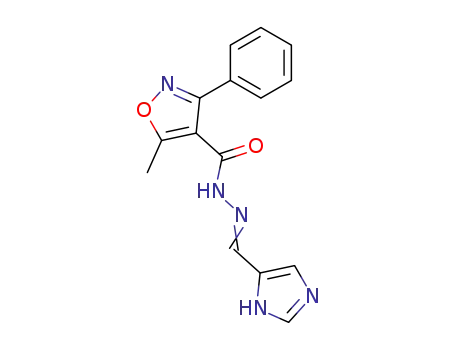
5-methyl-3-phenyl-isoxazole-4-carboxylic acid (1(3)H-imidazol-4-ylmethylene)-hydrazide
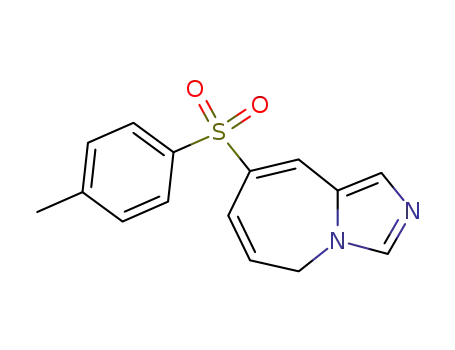
8-(4-Methylphenylsulfonyl)-5H-imidazo<1,5-a>azepin