Your Location:Home >Produts >intermediates >7531-52-4
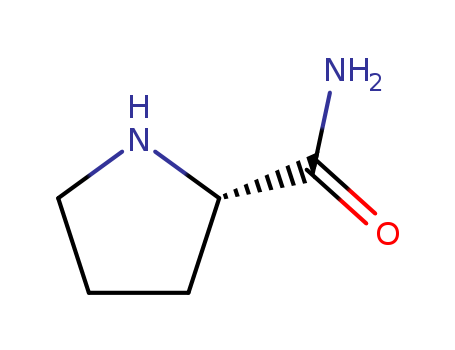

Product Details
|
Chemical Composition and Structure |
L-Prolinamide is an important intermediate compound used in the synthesis of polypeptides and chiral drugs. It is composed of a proline amino acid unit with an amide functional group. |
|
Mechanism of Action |
L-Prolinamide serves as a chiral ligand in asymmetric catalysis reactions, including Robinson cyclization and Aldol reactions. It participates in various organic transformations as a catalyst or co-catalyst, enhancing reaction rates and product yields. |
|
Production Methods |
L-Prolinamide is synthesized from L-proline through a series of chemical reactions, including esterification and ammonolysis. It is produced on an industrial scale using proline as the raw material. |
|
Analysis Method |
Liquid chromatography has been developed as an analytical method for detecting L-Prolinamide and its derivatives. This method allows for accurate and sensitive quantification of L-Prolinamide in various samples. |
|
General Description |
L-Prolinamide is a versatile and efficient organocatalyst, particularly valued for its role in asymmetric aldol reactions, where it enables high enantioselectivity (up to 99% ee) and diastereoselectivity (anti/syn ratios up to 99:1). It is recoverable and reusable with minimal loss in performance over multiple cycles, making it suitable for large-scale industrial applications. Additionally, derivatives of L-prolinamide have been utilized in the rational design of chiral acyl transfer catalysts, such as DMAP-N-oxides, for dynamic kinetic resolution, further demonstrating its broad utility in synthetic chemistry. |
|
Definition |
ChEBI: The carboxamide derivative of L-proline. |
InChI:InChI=1/C5H10N2O/c6-5(8)4-2-1-3-7-4/h4,7H,1-3H2,(H2,6,8)/p+1/t4-/m1/s1
-
-
The invention provides a preparation met...
The invention discloses a synthesis meth...
The invention belongs to the field of or...
In this study, bornyl- and cytisine-base...
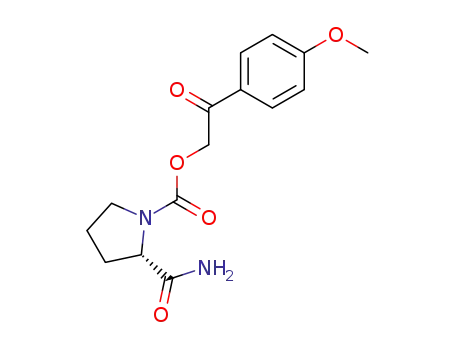
(S)-2-Carbamoyl-pyrrolidine-1-carboxylic acid 2-(4-methoxy-phenyl)-2-oxo-ethyl ester

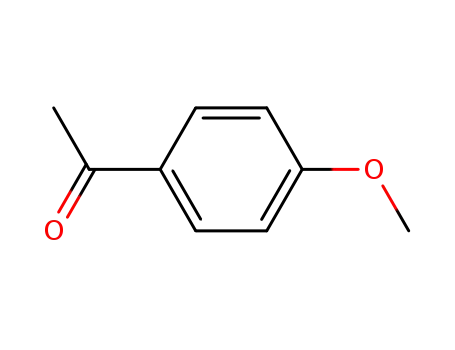
1-(4-methoxyphenyl)ethanone

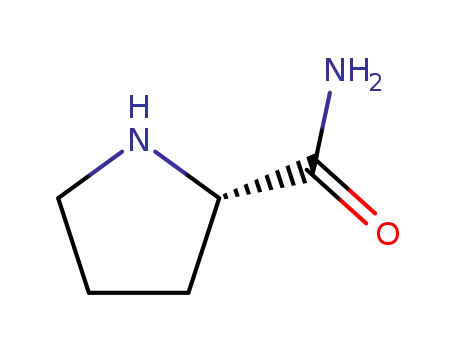
L-prolinamide
| Conditions | Yield |
|---|---|
|
Irradiation;
mild conditions;
|

L-proline methyl ester monohydrochloride


L-prolinamide
| Conditions | Yield |
|---|---|
|
With
ammonia;
In
methanol;
|
91% |
|
With
ammonia;
In
methanol;
at 0 - 20 ℃;
for 15h;
Reagent/catalyst;
Large scale;
|
85% |
|
With
ammonia;
In
methanol;
at -25 ℃;
for 96h;
Sealed tube;
|
55% |
|
With
ammonia; butan-1-ol;
at 20 ℃;
for 10h;
|
|
|
With
ammonia;
In
methanol;
Heating;
Large scale;
|
18 kg |
|
With
ammonia;
at 0 ℃;
for 26h;
under 2250.23 Torr;
Time;
|
15.8 g |
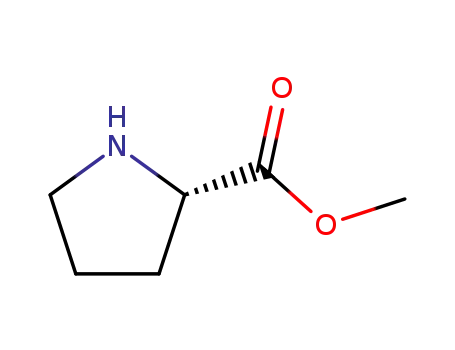
methyl (2S)-pyrrolidine carboxylate
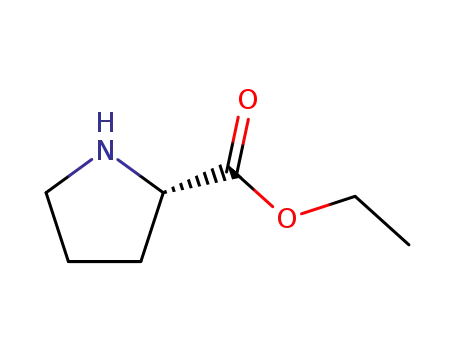
ethyl (2S)-pyrrolidine-2-carboxylate

(S)-2-Carbamoyl-pyrrolidine-1-carboxylic acid 2-(4-methoxy-phenyl)-2-oxo-ethyl ester
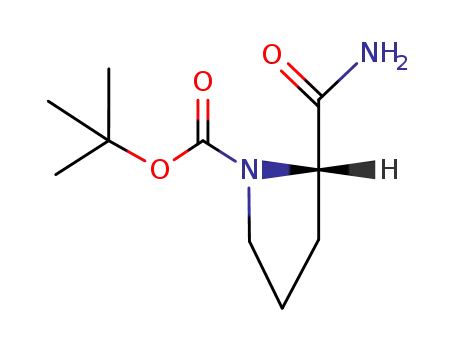
(S)-tert-butyl 2-carbamoylpyrrolidine-1-carboxylate
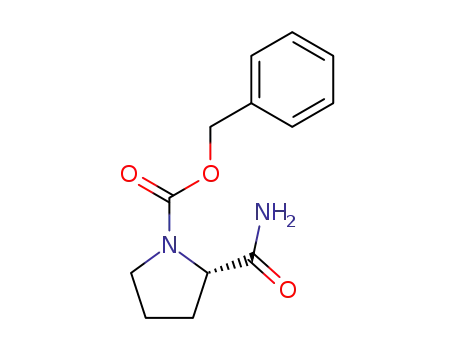
Z-Pro-NH2
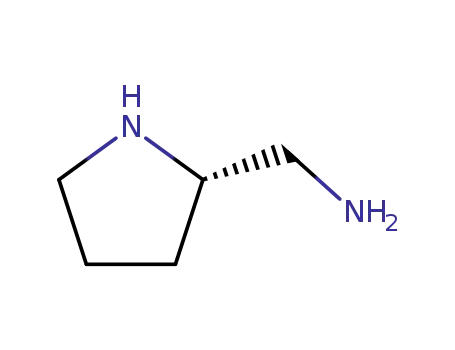
(S)-2-Aminomethylpyrrolidin
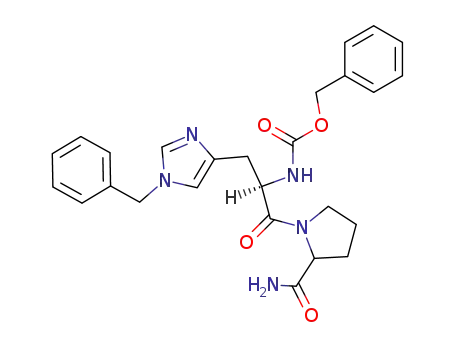
Z-D-His(CH2Ph)-L-Pro-NH2
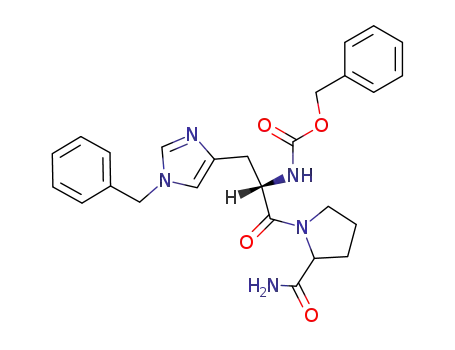
Z-L-His(CH2Ph)-L-Pro-NH2
CAS:23363-35-1
Molecular Formula:C27H30O13
Molecular Weight:562.527