Your Location:Home >Produts >intermediates >618-32-6
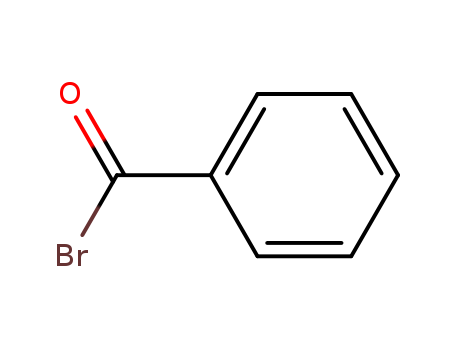

Product Details
|
Synthesis Reference(s) |
Tetrahedron Letters, 20, p. 3809, 1979 DOI: 10.1016/S0040-4039(01)95530-3 |
|
General Description |
Benzoyl bromide is a versatile reagent which causes the benzoylation of ethers. |
InChI:InChI=1/C7H5BrO/c8-7(9)6-4-2-1-3-5-6/h1-5H
Ternary bismuth halides are interesting ...
Potassium bromide mediated cross-dehydro...
A practical direct method for the direct...
A facile method for the direct synthesis...

dibenzyl ether

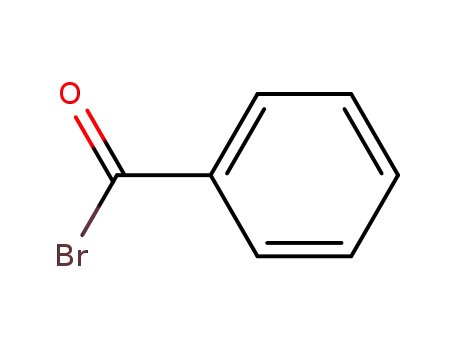
Benzoyl bromide

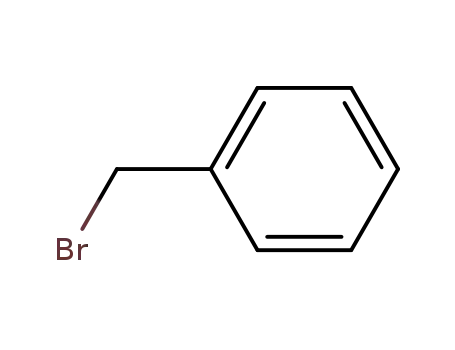
benzyl bromide
| Conditions | Yield |
|---|---|
|
With
bromine;
|

dibenzyl ether

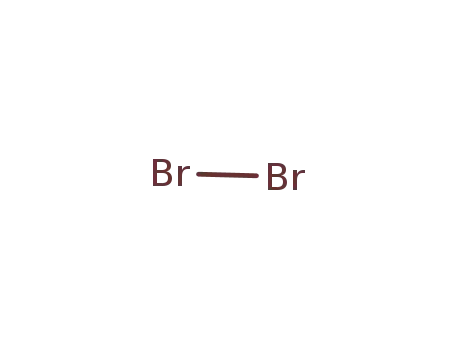
bromine


Benzoyl bromide

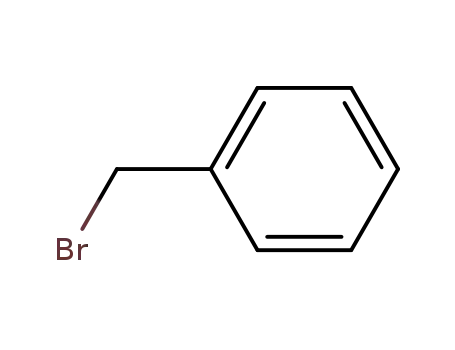
benzyl bromide
| Conditions | Yield |
|---|---|
|
|
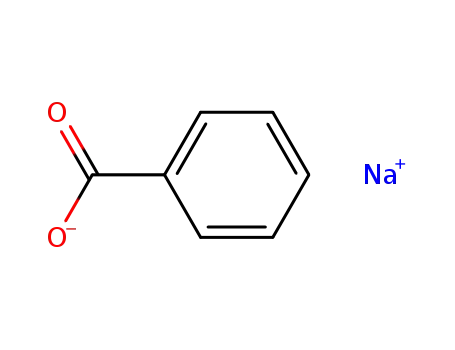
sodium benzoate
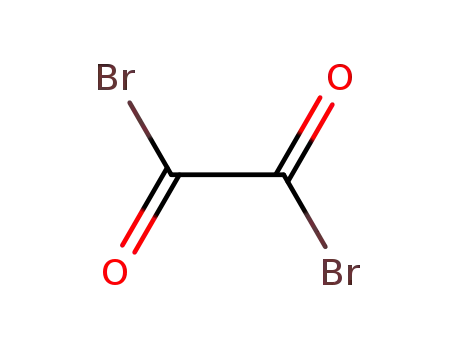
Oxalyl bromide

dibenzyl ether
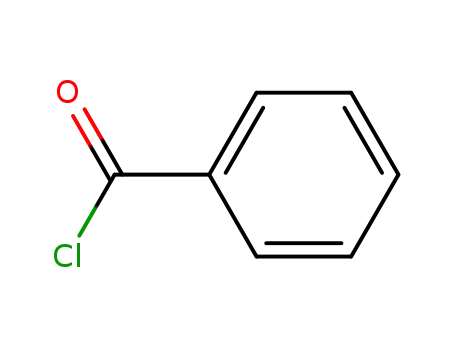
benzoyl chloride
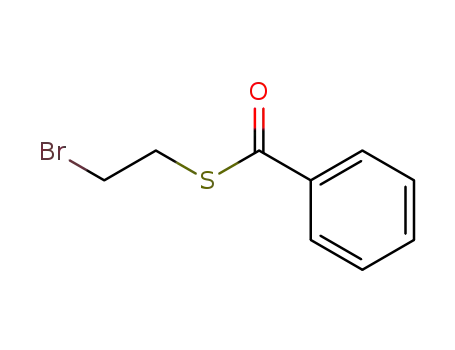
S-(2-bromoethyl) benzothioate
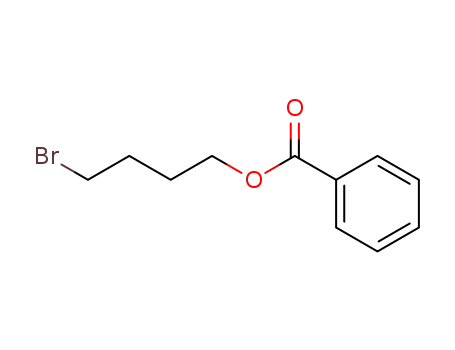
benzoicacid 4-bromo-butyl ester
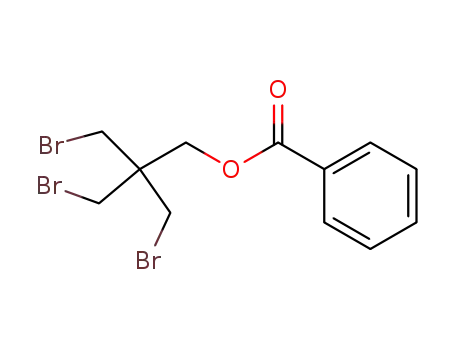
benzoic acid-(3-bromo-2,2-bis-bromomethyl-propyl ester)
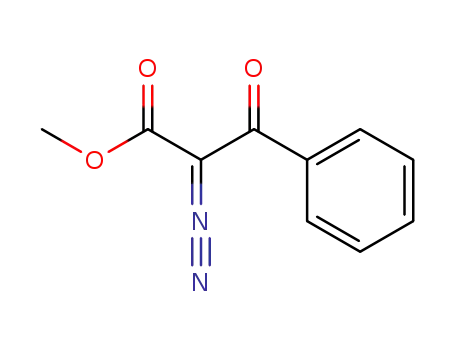
2-diazo-3-oxo-3-phenyl-propionic acid methyl ester
CAS:82398-30-9
Molecular Formula:C16H19 N . Cl H
Molecular Weight:261.794